Digital transformation is not a new concept. But pharma organisations should be continually reviewing whether their approach to digitalizing lab processes is working and meeting their objectives, and what they can do differently if it isn’t. The technology in platforms such as laboratory information management systems (LIMS) and informatics solutions like electronic lab notebooks (ELNs) available today are more sophisticated than ever, having benefitted from years of innovation. But there are still some gaps that need filling; labs are now looking at how data flows are connected across multiple disciplines, with the aim of creating a holistic view of the organisation’s lab data.
Big pharma and biotech companies are keen to capitalise on the machine learning (ML) and artificial intelligence (AI) revolution, as well as the potential of data mining. The investment in these technologies will undoubtedly change the way that drugs are manufactured and delivered in the future. But to understand how informatics will likely shape the future of pharma, we must take a look at how we got here, and the advances in lab technology and digital solutions that enable the transformations we are seeing today.
Disruptive tech in the beginning
For many, the adoption of a key lab system such as LIMS or ELN signalled the start of digital innovation. The days of using paper notebooks to record and store data are, for the most part, long gone, and technology has been gradually expanding lab productivity ever since. In the early days, however, lab-based organisations were often keen to implement informatics tools and processes for efficiency gains in the lab, rather than evaluating how they would contribute to the wider business’ goals and objectives.
Over the past few decades there have been a number of catalysts that have driven lab informatics upgrades and replacement systems. Such a key event was the introduction of the Title 21 Code of Federal Regulations Part 11 (21 CFR Part 11), which outlines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records.1In the heavily regulated pharma and biopharma industry, having informatics solutions in place that supported 21 CFR Part 11 and ensured data were secure and validated was vital. This triggered the evolution of technologies like LIMS, ELNs and chromatography data systems(CDS)to support improved audit trails and electronic signatures (legally binding e-signatures).
More recently, the wider topic of data integrity within systems and processes has been a driving force for implementing or accelerating lab informatics solutions within the pharma and biopharma industry. Outside of the regulated space, general requirements such as ISO/IEC 170252are reinforcing the drive for data integrity. ISO/IEC 17025 accredited labs are considered to operate competently and generate valid results and, in many cases, accreditation is a requirement from suppliers or regulatory authorities. Information systems are often critical in meeting these requirements, so organisations benefit from considering their quality systems and digital processes in tandem when looking to update or implement new informatics.
Modern data needs modern solutions
Moving on to current drivers for digital transformation, it is hard to ignore the push for greater collaboration and reuse of data – whether it’s within an organisation, externally or, as is often the case, both.
Labs across all sectors are striving to produce FAIR data (Findable, Accessible, Interoperable, and Reusable) to increase the value they derive from their data. These labs will find doing so more straightforward with solid digital processes in place.
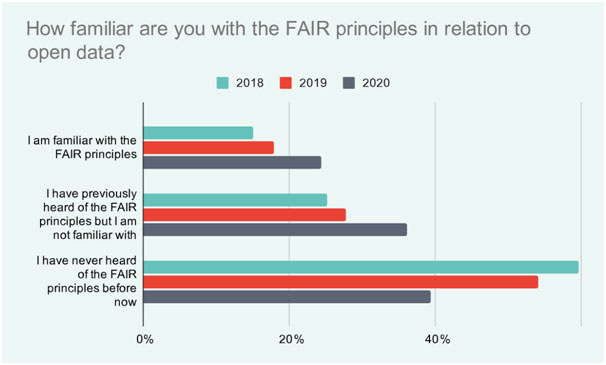
The importance of FAIR data is growing as more researchers become aware of the advantages. A recent survey found that in 2018, nearly 60% of researchers surveyed had never heard of FAIR principles, whereas in 2020 this had reduced to 39% (Figure 1).3Having lab informatics in place that link the entire lab workflow will make the process of submitting data to central repositories possible, which in turn will facilitate data sharing and re-use.
The cloud and COVID-19
Universal connectivity – where a single system can be deployed across the entire lab for significant productivity gains – is an essential building block in powering digital transformation. Lab technology is evolving quickly to support this, and the transition from client PCs connected to application servers to cloud-based computing has been at the centre of this move to universal connectivity.
To build an informatics system that meets today’s data compliance and security requirements, pharma and biotech companies need suppliers with a deep understanding of industry-specific technology within the lab environment. The right software and connectivity can help support the lab’s workflow with a seamless, easy to use, high-performance solution; combining cloud-based architecture with lab-specific functionality delivers a powerful new approach.
The ease with which cloud native applications and storage enable remote working on instruments and informatics systems, has shown to be crucial for business continuity throughout the COVID-19 pandemic. The restrictions on personnel operating in labs and offices spurred the upgrade or installation of new informatics systems to enable new ways of working. Many labs had some digital processes in place already but were not set up for the entire workflow to be available externally. At the same time, the pandemic accelerated the growth of many small- to medium-sized pharma organisations, exacerbating the need for solutions that could help improve data flow.
The connected lab of the future
Lab directors, IT professionals and the lab informatics industry are inarguably turning their sights towards digital lab transformation. Although the goals haven’t changed, the ways in which we are achieving this transformation are constantly evolving. For instance, AI and ML have the potential to transform day-to-day operations in the lab, maybe through voice recognition facilitating notetaking, or reducing the costs of lengthy clinical trials by using historical data to predict issues and prevent them before they occur.
The next level of informatics development is now leveraging AI and ML to propel digital science forward and to revolutionise the everyday life of scientists. By delivering lab informatics functionality in a secure, fast and efficient manner, AI and ML are helping organisations gain optimum value from lab effort. In terms of pharma research and development, this translates into scientists having more time to concentrate on core, value added activities, and bringing next-generation therapeutics to market faster.
For more information about lab informatics consultancy, please visit https://scimcon.com/
References:
- “CFR – Code of Federal Regulations Title 21”. U.S. Food & Drug Administration. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-11 [Accessed 18.01.22].
- ISO/IEC 17025 Testing and Calibration Laboratories. https://www.iso.org/ISO-IEC-17025-testing-and-calibration-laboratories.html [Accessed 18.01.22].
- Hahnel, M., McIntosh, L. D., Hyndman, A., et al. (2020): The State of Open Data 2020. Digital Science. Report. https://doi.org/10.6084/m9.figshare.13227875.v2
About Geoff Parker:
Geoff Parker is co-founder of Scimcon Ltd, an informatics consultancy that provides information systems consulting specifically for lab-based or clinically driven life sciences companies. With 20+ years of experience in working within life science and informatics projects, Geoff oversees the growth of Scimcon and also acts as a lead consultant within the company.
Contact email: gparker@scimcon.com


















