Extending the life cycle of cancer drugs
In 2022, there will be an estimated 1.9 million new cancer cases diagnosed and 609,360 cancer deaths in the United States.
Cancer affects large numbers of people each year, including many close to you and I. Just as cancer affects large numbers of people, cancer drugs make up a large portion of the drug (pharmaceutical) industry’s revenue, and there has been remarkable progress seen the effectiveness of cancer drugs over recent decades, with new types of treatments being proposed and approved by the FDA every year. Data about cancer and ant-cancer drugs can be found here: https://phrma.org/resource-center/ and https://www.cancer.org/research/cancer-facts-statistics.html
There are many reasons for why cancer remains a top challenge in drug development. A big reason is that a patient can be resistant to drugs based on nuances such as his/her genetic mutations. To complicate things, resistance can be acquired over time – such that cancer drugs may lose effectiveness over a 5–10 year period. This leads to (sub-)populations of cancer patients who are underserved.
While effected populations need more efficacious therapeutic treatments, parallel pharma companies need ways to extend the life cycles of their FDA-approved cancer drugs. In pharma, commercial leaders (those who sell the drugs) are very focused on the Life Cycle Management (LCM) of their drugs. They have sales targets to sell approved drugs for long as possible, and they are continuously looking for ways to extend the life cycle of their portfolio. The diagram below shows the end-to-end lifecycle – from drug development and sales in the industry, through to the commoditization of drugs as generics are made available.
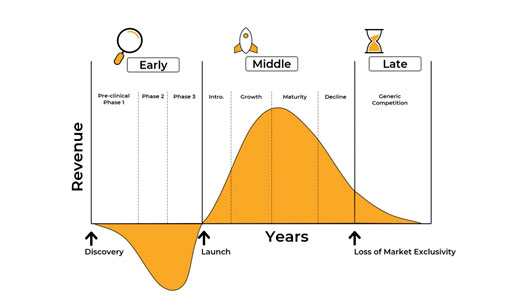
https://www.thepharmamarketer.com/knowledge/pharmacetuical-product-life-cycle
Pharma companies often turn to combination drugs to enhance the LCMs of their popular cancer drugs. If the FDA has already approved each of the drugs and they are in similar molecular pathways, then the time-to-market for the combination therapy can be relatively fast with manageable costs. So, the incentives are high for commercial leaders to identify novel combination therapy treatments in cancer.
Correlation is not causation
“Data can tell you that the people who took a medicine recovered faster than those who did not take it, but they can’t tell you why. Maybe those who took the medicine did so because they could afford it and would have recovered just as fast without it.”
- Judea Pearl
The life sciences industry is full of innovative projects using traditional statistical methods, such as traditional machine learning and AI. These correlative methods (which are hypothesis-free) have led to the discovery of many novel drugs. But they have limitations. Namely, that they cannot be used to identify causation. This creates risks for scientists.
Causation (modeled using Causal AI) allows scientists to hypothesize potential mechanisms behind observed disease progression, not just predicting the progression itself. Armed with this information, scientists can develop actionable hypotheses about what can be done to improve the cancer treatment. Without getting into the details, key points of Causal AI that differentiate it from traditional correlative (machine learning and AI) techniques are to:
- Leverage known biological knowledge as a starting point for the model.
- Model the biological system as a network.
Accelerating identification of combination therapy treatments
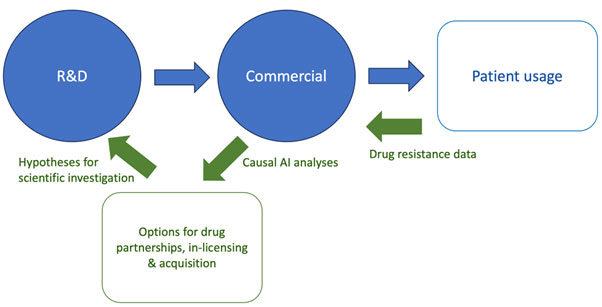
The diagram below shows how Causa AI activities (the green arrows) can rapidly and cost-efficiently analyze data from the traditional workflow (the blue steps) to enable new treatment ideas to be developed and tested. Pharma companies can leverage drug resistance data to hypothesize molecular pathways to target with additional drugs.
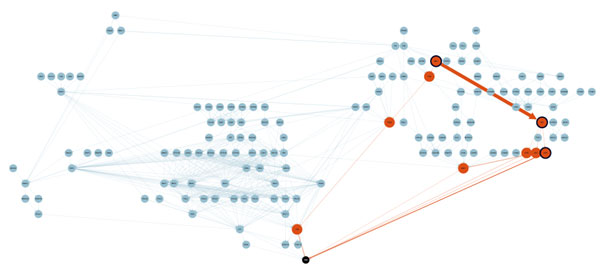
Take a look at the diagram below. The nodes and lines represent molecular pathways that are understood by cancer biologists as relevant to (in this example) ovarian cancer. Drug discovery scientists spend their days drawing and thinking about such diagrams.
In this example, AstraZeneca sells a drug to address ovarian cancer called AZD6738. Causal AI simulation (using publicly available data) showed that there is potential and significant resistance to AZD6738 where the orange nodes and lines are shown. And, through some simple investigation, it turns out that there is another FDA-approved drug (Dasatinib, sold by BMS) that could potentially enhance the overall effectiveness of AZD6738 by directly addressing the resistive pathways in target patients.
Voila! A scientific hypothesis has been formed for a combination therapy, for R&D to investigate (back to the diagram above).
To learn more about this example: https://www.incubate.bio/alasca
In conclusion
AI in itself will not fix cancer. But the smart application of explainable and actionable Causal AI methods will accelerate the identification of new combination therapies. This in turn will give confidence and business justification for pharma companies to rapidly investigate, develop and then offer new treatments with FDA-approved drugs. And the winners will be the patients.
BIO
Dr Raminderpal Singh is a recognized KOL in the techbio industry. He has over 30 years of global experience – leading and advising teams building computational modeling systems that are both cost-efficient and have significant IP value. His passion is to help early to mid-stage life sciences companies achieve novel biological breakthroughs through the effective use of computational modelling.
Raminderpal is currently the CEO and co-Founder of Incubate Bio – a techbio providing a service to life sciences companies who are looking to accelerate their research and lower their wet lab costs through in silico modeling. Incubate bio’s technology (ALaSCA) applies causal analysis and machine learning to uncover drivers in biological systems (such as protein networks), using multi-omics and phenotypic (and clinical) data. Raminderpal also advises several early-stage life sciences and healthtech startups.
Raminderpal has extensive experience building businesses in both Europe and the US. As a business executive at IBM Research in New York, Dr Singh led the go-to-market for IBM Watson Genomics Analytics. He was also Vice President and Head of the Microbiome Division at Eagle Genomics Ltd, in Cambridge.
Raminderpal earned his PhD in semiconductor modeling in 1997. He has published several papers and two books, and he has twelve issued patents. In 2003, he was selected by EETimes as one of the top 13 most influential people in the semiconductor industry.
For more: http://raminderpalsingh.com


















