While human beings tend to adopt many different ideas over time, one that remains a mainstay under all situations is our idea of growth. You see, no matter what happens, human beings are always looking for ways to grow on a rather consistent basis. By doing so, we end up giving ourselves a prime at some significant milestones. However, if we were to sit back and assess these milestones in hindsight, we probably won’t find anything as significant as technology. Technology enjoys such an unprecedented position in our lives due to factors that go deeper than just its ingenious skill-set. For instance, they revolve a lot around how the creation, regardless of the variety in play, was so successful with impacting each and every area on our spectrum. The stated element, as you can guess, would go to produce a host of beneficiaries in its own right, and a big one among them will be our healthcare sector. Technology-healthcare collaboration stands out from the rest because it came at a time when the latter was really desperate for a change. The change that followed, of course, placed the sector on a whole new trajectory. Rejuvenated on the back of new-age methodologies, the healthcare sphere was able to win over the masses again. In fact, this very dynamic is still running the show, and one recent FDA approval should only bolster it further.
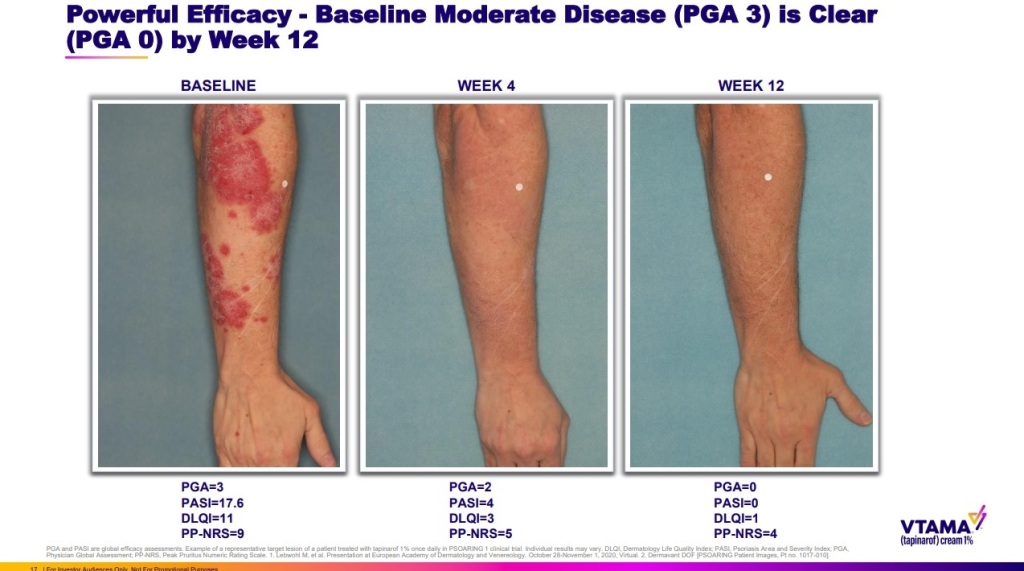
Roivant Sciences has officially bagged FDA’s approval for a drug candidate, which is purposed around treating inflammatory skin condition, plaque psoriasis. It’s a pivotal moment from the overall standpoint, as so far, topical steroids have largely headlined the treatment prescription for the condition. According to certain reports, these steroids can lead to thinner skin over time. Furthermore, they can also cause additional complications, in case you use them on sensitive areas. Roivant’s candidate, on the other hand, proposes a 100% steroid-free approach. Designed to block inflammatory proteins called cytokines, the newly-approved drug, which will be marketed under the name “Vtama”, is supposed to be applied once every day. Interestingly enough, it won’t just treat the disease in question, but it will also focus on reducing oxidative stress that contributes to various inflammatory skin conditions.
While the drug falls within the umbrella of Roivant, it was actually acquired and developed by its subsidiary in Dermavant Sciences.
“We are delighted with our FDA-approved label for VTAMA cream, which is for adults with psoriasis, regardless of disease severity, and with an unlimited duration of use. In anticipation of today’s approval, we have a fully built commercial infrastructure in place, and I am excited to say we will have product in the channel in the first week of June. As the first and only approved drug in its class in the U.S.,” said Todd Zavodnick, Chief Executive Officer of Dermavant Sciences.
The FDA’s approval for Vtama comes after two separate studies, which featured 1,000 patients in total. As per the available details, in both the studies the drug was able to show meaningful progression after 12 weeks of treatment in comparison to an alternative that had no active ingredient.