We spend a sizeable amount of time in trying to understand what components shape our lives, and most importantly, how they do it. This helps us big time with chalking up our unique growth trajectory, while also ensuring that we follow up on it in a productive manner. Now, even though, at its core, the setup is different for each individual, we find ourselves, more often than not, working towards a similar goal of making the world a better place. There can be some significant differences in our approaches, but the driving force remains shared to a larger extent. If anything, we have even turned these little differences into something advantageous. The said variance, as you can guess, has enabled us to touch upon many meaningful parts of the spectrum, so it’s almost a given that such a scenario will yield a ton of by-products, but the biggest one to emerge here was technology. In hindsight, you can easily see how technology altered the course of history by literally reinventing the world’s identity. The tweak, however, was a result of things happening at the granular level. For instance, every major sector out there went through some major transformations to be able to work within a highly optimized environment. The call to undertake something of this sort was huge by all means, except it started paying off dividends right away. One area where they happened to appear in spades was the medical sector. As a result, we have witnessed the core meaning of health evolve drastically. Nevertheless, the landscape seems set for scaling up yet again, as metaMe Health gets regulatory nod for a groundbreaking treatment.
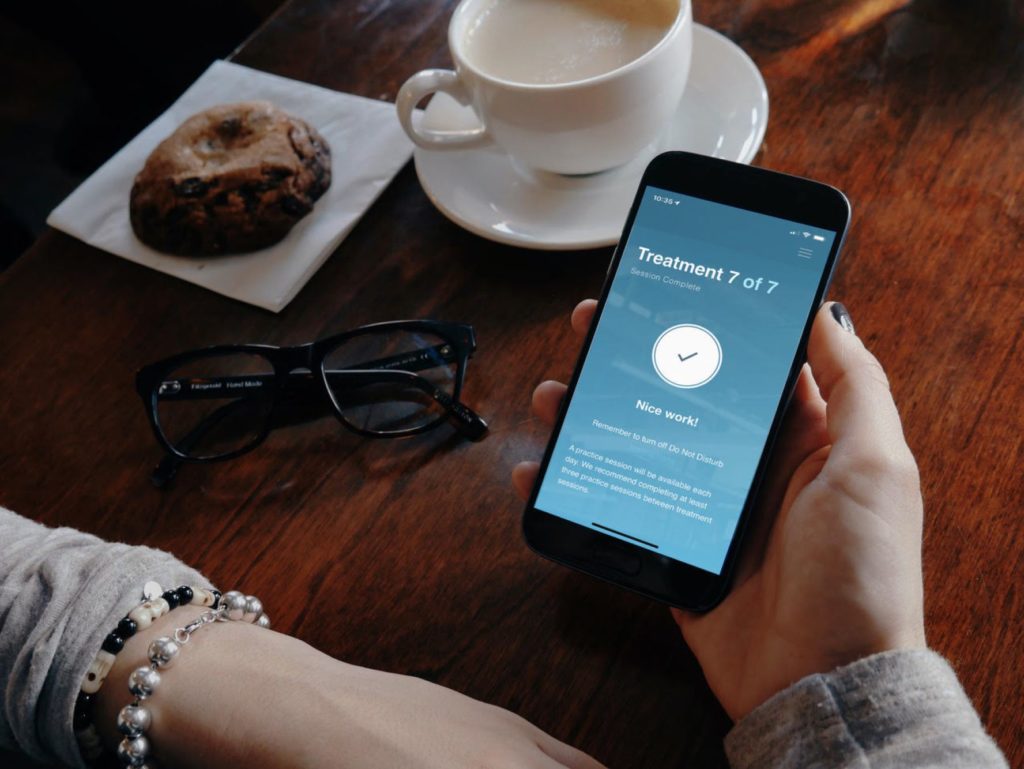
FDA has officially cleared metaMe Health’s prescription-only digital therapeutic called Regulora, which is designed to treat abdominal pain associated with irritable bowel syndrome (IBS) in adults aged 22 years or older. Regulora essentially functions in the form of a 3-month self-administered course. Inspired by gut-directed hypnotherapy, the therapeutic structures your treatment in a way that sets you up for seven 30-minute sessions delivered through a mobile app, therefore boasting an immense overall accessibility.
“The FDA’s clearance of Regulora, our first PDT, is a major achievement for the company,” Tim Rudolphi, CEO of metaMe Health, said in a statement. “We are eager to expand access to this proven therapy to patients with all three subtypes of IBS.”
Regulora has already proved itself in a supervised setting, where the therapy was tested in over 362 patients suffering from IBS symptoms. As per the results shared by metaMe, 68% of patients reported complete satisfaction, whereas a whopping 87% claimed that they are willing to recommend the digital therapeutic to others. Regulora is penciled in to hit the market in the first half of 2022 and will be available for both Apple, as well as Android users.