Introduction
The U.S. FDA Drug Supply Chain Security Act (DSCSA) is a significant regulatory framework designed to ensure the safety and security of the pharmaceutical supply chain in the United States. This article outlines the key components of DSCSA, the challenges faced by industry stakeholders in complying with the act, and how advanced technologies like Microsoft Copilot can aid in navigating these challenges.
DSCSA will surely impact PBMs. The regulatory environment surrounding PBMs is continuously evolving, with new rules and standards being introduced to address industry challenges. Keeping abreast of these changes is crucial for PBMs to ensure compliance and adapt to new requirements that impact their operations.
Background on DSCSA
The DSCSA was enacted by the U.S. Congress to enhance the security of the drug supply chain and to protect consumers from exposure to harmful drugs.
It mandates that pharmaceutical companies, including manufacturers, repackers, wholesalers, and dispensers, adhere to stringent tracking and traceability requirements. The act also necessitates the standardization of product identifiers, including the National Drug Code (NDC), serial numbers, lot numbers, and expiration dates, to facilitate traceability at various levels of the supply chain, from individual packages to pallets.
Challenges in DSCSA Compliance
Compliance with DSCSA is complex and presents significant challenges for stakeholders. The variability in regulations depending on the stakeholder’s role—be it a manufacturer, repacker, or dispenser—complicates the process of finding relevant regulatory information. The FDA’s existing search tools for regulatory guidance are often ineffective, with metadata-based searches leading to inconsistent results. For instance, searching for “DSCSA” on FDA.gov yields only a few relevant hits, illustrating the difficulty in accessing comprehensive regulatory information.
Comparison of DSCSA with EU FMD and India DAVA
US FDA drug supply chain security measures differ from similar regulatory frameworks in other regions, such as the European Union’s False Medicine Directive (FMD) and India’s DAVA system. The EU FMD focuses on preventing falsified medicines from entering the legal supply chain, requiring unique identifiers and anti-tampering devices on drug packaging.
US FDA’s DSCSA lack of a central supply chain security database will undoubtedly complicate the US drug supply chain compliance. Unlike EU’s FMD or India’s DAVA supply chains security measures, the DSCSA does not mandate a central repository, with each trading partner maintaining its system for tracking and traceability. India’s DAVA system, on the other hand, operates a centralized database with mandatory serialization for all levels of packaging, covering both domestic and export markets. A secure quasi public, quasi private cloud hosted databases would greatly simplify supply chain regulations so it’s puzzling why US FDA has chosen not to implement such an approach.
Nonetheless, DSCSA mandates become mandatory come November 27, 2024. The FDA recognizes the great challenges faced by drug industry stakeholders specially smaller last mile pharmacy drug dispensers and has instead created what’s known as ‘Wee’, i.e. waivers, exceptions and exemptions. FDA will consider each waiver, exception or exemption in case by case basis but does not guarantee and automatic grant or approval, therefore industry stakeholders should not consider such an application as pass and release from meeting DSCSA regulations.
Role of Microsoft Copilot in DSCSA Compliance
A big concern for drug industry stake holders to fulfill DSCSA requirements apart from being complex is the lack of a robust search system hosted at th FDA.Gov website. FDA’s search is meta-data based meaning that if one’s search keywords do not match the FDA assigned tags, the search will miss credible search results that maybe contained inside an actual PDF or file, i.e. the US FDA.Gov search is NOT full-text searchable.
However, there’s a new (technology) ‘sheriff in town’ and the` badge reads ‘Copilot’
Microsoft Copilot, powered by OpenAI’s ChatGPT-4 AI/ML, offers a promising solution to the challenges of DSCSA compliance. By integrating advanced AI and machine learning capabilities, Copilot can enhance the search and retrieval of regulatory information from FDA databases, overcoming the limitations of traditional search methods.
A tool such as a FDA-site data augmented Copilot allows one to deploy artificial language and machine learning technology against regulatory content using natural conversational language in English by default but can be extended to converse in foreign languages, i.e. a great boon to foreign actors that participate in th US local drug markets from manufacture, processing or drugs exports consumed in the US healthcare scenario.
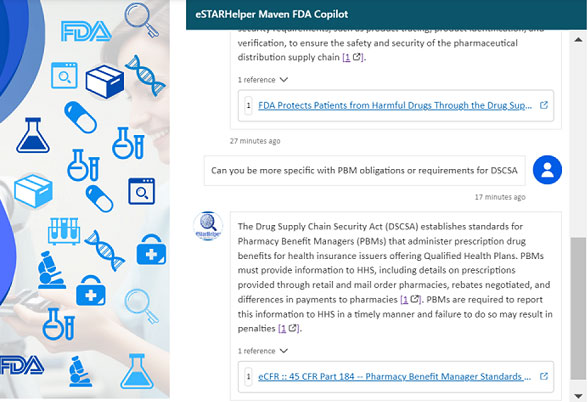
With Microsoft Copilot on FDA, one can perform full-text searches within FDA guidance PDF documents and 21 CFR regulations, ensuring that stakeholders can access the precise information they need without relying on often inadequate metadata tags. Furthermore, Copilot can be customized to integrate enterprise-specific data, such as SOPs and quality policies, providing a tailored solution for compliance verification and regulatory preparedness. Drug Supply Chain Security Act (DSCSA) | FDA
Conclusion The DSCSA represents a critical advancement in securing the U.S. pharmaceutical supply chain, but its complexity poses significant challenges for compliance. The introduction of tools like Microsoft Copilot offers a new approach to overcoming these challenges, leveraging AI to streamline the process of finding and verifying regulatory information. As the pharmaceutical industry continues to evolve, such technological innovations will be essential in ensuring continued compliance and safeguarding public health.



















