Diversity, in the realm of medical devices, emerges as a crucial and multifaceted consideration, setting it apart from other healthcare products. This distinction becomes evident in various facets such as clinical research, marketing, and supply chain dynamics. Unlike the pervasive nature of human factors diversity across all healthcare products, medical devices introduce additional layers, creating a broader and more intricate landscape for consideration. The term diversity, in this context, transcends mere variability; it extends across different dimensions, acknowledging individual differences. In the realm of medical devices, diversity operates on two primary planes – human factors diversity and device factors diversity. While interactions occur within each plane, inter-planar attributes further complicate the relationship, creating a nested and intricate system.
To maximize the utility of medical devices in healthcare, a robust synergy between human factors and device factors is essential. For instance, many medical instruments are traditionally designed for right-handed operation, overlooking the approximately 10 to 12% of the global population that is left-handed, including doctors. This oversight underscores the need for careful management of device diversity to address the compounded effects of these intricacies.
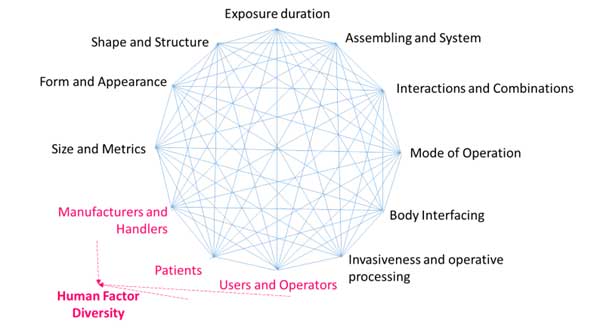
Figure 1: Attributes of device and human factor diversity and their interaction
Among the diversity factors, human factor diversity receives significant attention, whereas medical device diversity is often understated. Research by Adusumilli et al. highlights low sensitivity concerning the laterality of instrumentation in both simple and critical surgical areas, shedding light on the insufficient emphasis on medical device diversity. Moreover, training on laterality in early medical education is not readily available, further emphasizing the need for a comprehensive approach.
Human Factor Diversity
According to the World Health Organization (WHO), human factors encompass the understanding of interactions among humans and other elements of a system. This field applies theoretical principles, data, and methods to optimize human well-being and overall system performance. Human factors research delves into human behavior, abilities, limitations, and characteristics, applying this information to design tools, machines, systems, tasks, and environments for safe, effective, and comfortable human use.
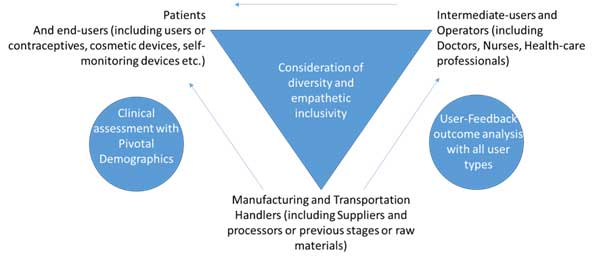
Figure 2: Human Factor Diversity and empathy-based inclusivity and remediation
The physical, cognitive, and organizational domains constitute the three pillars of human factors in healthcare, as outlined by WHO. While the physical domain pertains to body interactions and design, the cognitive domain focuses on mental processes, and the organizational domain involves collective interactions with technology. In the context of medical devices, the physical domain extends to factors like age, race, sex, sexual orientation, body size, ability, disability, dominant hand use, culture, diet, habitat, literacy, language, and attire. Each of these attributes requires careful evaluation and inclusive remediation at points where devices interface with humans: patients, users and operators, and those involved in the manufacturing and transportation processes.
Patient Diversity
Patient diversity emerges as a highly variable factor, contributing to the complexity of human factors in medical devices. Each patient introduces multi-axial individualistic variations based on demographic and co-morbidity references. Inclusive pivotal studies, as mandated by regulatory requirements, must encompass subsets representing women and racial and ethnic minorities. However, compliance to inclusivity subsets in reported studies often falls short, necessitating a more thorough approach to assess the impact of population diversity and its interaction with medical device diversity.
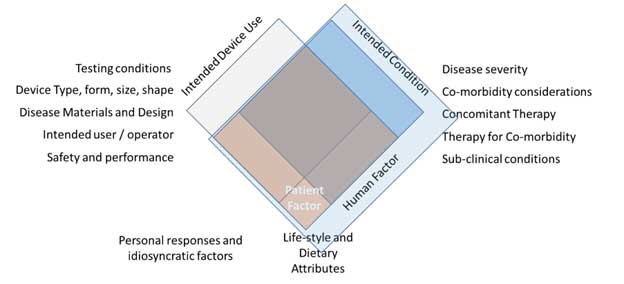
Figure 3 Human Factor Diversity and Inclusivity – Patient perspective
Inclusivity from the patient perspective involves incorporating diverse subsets in pivotal studies or post-market clinical follow-up data collection. Even in cases where a device does not directly impact clinical outcomes, the effects of patient and disease diversity on subordinate or delivery outcomes must be rigorously evaluated. This comprehensive approach ensures that all potential conditions influencing the device’s intended outcome are assessed.
User and Operator Diversity
The diversity among users, especially operators, remains a neglected aspect in the consideration of human factors in medical devices. Operators, including doctors, nurses, technicians, lay-users, patients, and caregivers, bring diverse educational backgrounds and specialties. Operator diversity significantly influences device outcomes, making it imperative to identify the impact of human factors and usability engineering on medical device use and pinpoint safe and unsafe use zones.
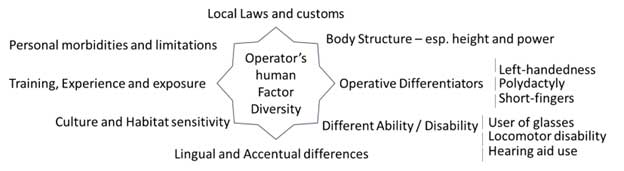
Figure 4 Operator’s Human Diversity Factor
The User and Operator Diversity framework, as advocated by the MHRA’s Human Factors and Usability Engineering guidance, seeks to apply usability engineering processes to identify, assess, and mitigate potential safety risks for patients and users. This approach is crucial in enhancing device design and minimizing incidents through corrective actions.
Diversity Management beyond Inclusion and Usability Engineering
In managing diversity for medical devices, a shift beyond conventional inclusivity management is required. This involves a broader strategy encompassing knowledge, understanding, application, and skills of device stakeholders in the context of device diversity. Effective communication, responsibility determination, mapping equality and diversity with device use, creating a usability group, and implementing education or mentorship programs form the pillars of this strategy.
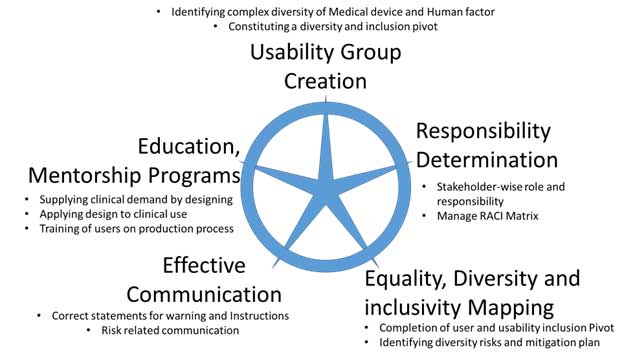
Figure 15 Diversity Management for Medical Devices
One pivotal aspect of diversity management involves forming a usability group, comprising personnel from various organizational levels and representing the entire spectrum of human factor diversity. This group plays a central role in creating scenarios around each intended use of the device, its operative steps, and functions throughout the device’s lifecycle. By determining roles and responsibilities through a RACI matrix, the usability group ensures a comprehensive analysis of diversity.
In some instances, consultation with local representatives from various countries or stakeholders like ethics committees may be necessary to understand regional nuances and enhance inclusivity. External stakeholders, such as investigators and ethics committees, become vital contributors to the diversity mapping process, especially in the realm of clinical research. Upon completion of diversity mapping and the usability inclusion pivot, the next step involves data collection and risk management. This data-driven approach identifies diversity risks and mitigation plans, facilitating equality, diversity, and inclusivity mapping. The clarity obtained from this process informs action plans for addressing diversity through inclusivity, encompassing mentoring plans, evaluation of skill-based changes, and effective communication of diversity requirements.
In essence, the journey through diversity in medical devices necessitates a holistic approach that transcends traditional inclusivity measures. By recognizing the intricate interactions between human factors and device factors, stakeholders can navigate the complexities, ensuring the optimal use of medical devices across diverse populations and contexts.


















