Last year, the US Food and Drug Administration (FDA) issued multiple warning letters to manufacturers of lower quality thermal imaging devices for improper use and unfounded claims. It confirmed that some low-cost devices used in schools and public places record temperatures that are extremely inaccurate and may be dangerously ineffective. These actions will help consumers recognize more accurate infrared (IR) thermographic devices that have undergone rigorous testing and meet FDA clearance standards.
The Chief Medical Officer and director of the Office of Product Evaluation and Quality in FDA’s Center for Devices and Radiological Health stated that “thermal imaging … can determine if someone has an elevated temperature, which can be an important risk management tool during the pandemic when used properly.” However, William Maisel, M.D., M.P.H. went on to say that, “improper use and marketing of thermal imaging systems may lead to inaccurate temperature readings and pose a potential danger to public health.”

Food and Drug Administration headquarters White Oak, MD
CQ-ROLL CALL, INC VIA GETTY IMAGES
The independent surveillance research organization IPVM conducted tests of thermal cameras and warned that some are “dangerously ineffective” and raised concerns that infected people pass through medical screening checkpoints and go on to spread COVID or other viruses. The organization shared its testing results with the FDA, who then responded by issuing warnings to manufactures for selling “unapproved, uncleared, and unauthorized thermal imaging systems.”
IR thermography has been widely used for security and industrial uses ranging from monitoring manufacturing equipment and cold storage systems to detecting the presence of intruders or even people carrying guns. Only a handful of IR devices were available to detect elevations in body temperature after having gone through the laborious regulatory approval process. Companies such as FLIR (now part of Teledyne), and Infrared Cameras Inc (ICI) have received FDA clearance to be used for rapid and convenient screening of individuals with elevated body temperature.
All that changed in the global scramble to respond to the COVID pandemic.
Within a matter of months after the start of the pandemic, the sale of telethermographic systems exploded. In an effort to address a potential shortage of available products, the FDA loosened the guidelines on the use of heat-sensing devices, allowing many manufacturers to market products for fever detection that were not designed or tested specifically for that use. Across the U.S., municipalities, public facilities, businesses, schools, hospitals and many other venues rushed to purchase and install systems that they believed would help them be able to rapidly identify people who had fevers and were at risk of COVID infection.
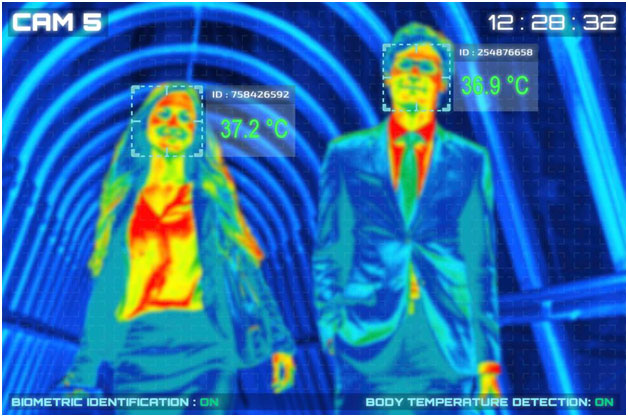
Infrared thermal camera GETTY
Accuracy and safety concerns caused scrutiny by watchdog groups such as IPVM. Among the hundreds of companies marketing devices to check when someone has a fever, many were found to be using unethical marketing practices or technologies that were not engineered use in any medical applications. Therefore, in March 2021, the FDA issued a public alert noting that “improper use of the systems may provide inaccurate temperature readings due to a variety of factors.” The same day the agency also issued “Warning Letters” to 13 thermal imaging device suppliers for “violations of regulatory significance,” specifically for technologies that claim to measure “multiple individuals’ temperatures simultaneously.”
Many of the handhelds sold recently as “fever detectors” are actually devices used to measure a change in ambient temperature or merely the presence of a warm body. They lack the instrumentation and precision necessary to detect changes in body temperature that would indicate a fever, even when used in a “point and shoot” application at close range. Options for fever screening that have been shown to offer the highest levels of precision and reliability are based on the use of infrared thermal camera technologies. Only a handful of companies such as ICI offer systems that have been specifically engineered to effectively identify elevated body temperatures remotely and have been cleared by the FDA.
Infrared temperature screening systems use thermal imaging cameras and software that is specifically developed to be able to read skin surface temperature and rapidly calculate an estimated core body temperature. If that temperature is above a preset range, in most cases the device will issue an alarm to indicate the confirmation of an elevated temperature. Only a few IR devices offer reliability and accuracy of up to 0.1 to 0.2 ℃.
As COVID continues to be a public issue and other potential infectious pandemics lurk, analysis of deficiencies in public safety responses will be a critical component in positioning governments and health systems to be better prepared to respond to similar future health challenges. Within this framework, the need to identify and apply the most effective technologies in testing, monitoring and patient care will continue to be essential. Efforts to respond to a surge in demand for products during a global pandemic must be balanced with strategies that assess these options carefully and embrace the most appropriate and proven-effective technologies.


















