It is not a revelation to anyone that healthcare and clinical trials have a very big problem when it comes to diversity, equity, and inclusion. Two striking examples are seen with cardiovascular disease, where only 3% of participants in cardiovascular trials identify as Black or African American, despite the higher risk of heart disease among Blacks or African Americans compared to other racial groups, and only 2% of participants in oncology trials identify as Black women, compared to 84% identifying as White women. This is regularly discussed everywhere from the boardroom to the clinic, by all stakeholders. Patient advocacy groups are finally getting clinical trial sponsors, Pharma, medical device companies, and regulatory agencies to publicly and formally acknowledge the problem and more importantly, recognize the need for all stakeholders to take action. This is an important step towards a more equitable healthcare system. But it is time to accelerate the health equity movement.
A year ago, the FDA released draft guidance on Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials. While this was a welcomed addition to the ongoing discussions for the need to diversify clinical trials, a draft guidance is only a recommendation. Without a mandate, the FDA cannot hold anyone accountable. And so the discussions continued on this “nice to have” aspect of health care that is critical for so many underserved and underrepresented populations. The passing of the 2023 omnibus spending bill gives us some additional hope that we can move from talking about the need for diversity to taking action; action mandated by law. However, action will have to wait until after the FDA issues the final draft guidance. In the meantime, the “nice to have” discussions continue.
Instead of waiting for the mandate, clinical trial sponsors can start to take action now. Innovations in digital health technologies are promising to transform healthcare, including patient engagement, workflow efficiencies, diversifying and increasing data collections, and ultimately improving patient outcomes. The increase in use and access of these digital tools also provides opportunities for improving health equity. These opportunities come from two perspectives: digital health product development and digital health product deployment. Inclusive strategies for digital health product development and deployment can translate to improving health equity in clinical trials, by alleviating some of the burdens associated with clinical trial participation and limitations with knowledge gaps. Through these opportunities tangible action-oriented resources that can be implemented today were created. There is no room for more excuses.
The primary incentive for improving health equity should be that it is the right thing to do, and a more diverse, equitable, and inclusive clinical trials system will improve healthcare for everyone. While this has not been sufficient for substantial progress to date, digital tools can begin to accelerate efforts. Digital tools have the potential to address many of the challenges facing traditional clinical trials, including low recruitment and retention rates, missed enrollment milestones, incomplete data collections, and extended time for trial completions. All of these affect the financial bottom line; this may be the incentive that will drive many stakeholders. Digital tools can also transform the clinical trials system in a way that can bring about sustained progress with advancing health equity. With digital tools, those designing and implementing clinical trials can provide greater transparency and begin to build trust; two elements that are important for moving away from transactional encounters.
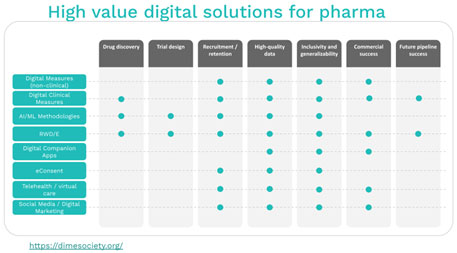
Some digital tools with the potential to transform clinical trials include artificial intelligence and machine learning (AI/ML), real-world data/real-world evidence (RWD/RWE), eConsent, clinical and non-clinical digital measures, digital companion apps, social media and digital marketing, on-demand video, virtual visits, and digital platforms and solutions. These digital tools are already being used in healthcare and clinical trials, however, true innovation to profoundly affect health equity requires more intentional efforts. The FDA guidance for diversity plans will call for specific actions to enroll and retain diverse populations.
Digital innovations will lead to disruption of the traditional clinical trials system. The interrogation of multiple RWD/RWE sources with AI/ML will open new avenues of identifying clinical trial participants and will allow for the inclusion of more diverse populations. Recruitment and retention methods will be enhanced with the use of social media and digital marketing, digital companion apps, eConsent, on-demand videos, and virtual visits. Clinical and non-clinical digital measures, RWD/RWE, AI/ML, and digital platforms will bring greater efficiencies to recruitment and retention, and health data collection and analytics. Several of these digital tools, including RWD/RWE, digital platforms, and virtual visits can be used to nurture partnerships with communities to develop trust and build a more inclusive clinical trial system.
Clinical trials need to move away from only academic medical centers and truly meet underserved and underrepresented populations “where they are”. This may include partnering with community health centers or implementing decentralized clinical trials. Powered by digital tools, decentralized clinical trials will reduce burdens on participants, increase trial efficiencies, decrease costs, and bring clinical trials to more populations who can benefit from participation. With over 400,000 participants, over 80% from populations underrepresented in biomedical research, the All of Us Research Program, the National Institutes of Health’s Precision Medicine initiative, is clearly demonstrating the success and advancement for health equity that comes with digital innovation and disruption.
The health equity movement is the paradigm shift those clinical trials and healthcare in general needs. Instead of making excuses or remaining stagnant, all stakeholders involved with designing and implementing clinical trials can take advantage of the rapid growth of digital tools. We can implement lasting change for a more diverse, equitable, and inclusive system to advance health equity for all.


















