Background
This review aims to identify which and how many of the most commonly prescribed drugs in the United States are associated with xerostomia and to provide an overview of safe management options.
Methodology
For identification of the most commonly prescribed medications in the United States, the medication expenditure panel survey was reviewed. Following the identification of the most prescribed medication, drug package inserts and clinical medication databases were utilized to obtain the incidence and documentation of xerostomia.
Results
Of the top 200 prescribed medications in 2019, more than half of the medications possess a risk for xerostomia. Of these medications that pose a risk for xerostomia, approximately 75% of these medications may be used chronically. Many of the medications are for conditions that may not benefit from discontinuing these medications, so safe xerostomia management options are needed. Over-the-counter (OTC) xerostomia options were identified with dosing and counseling recommendations to provide safe treatment options that do not interact with the commonly prescribed, and often chronic, medications that may be associated with medication-induced xerostomia. Prescription medications are discussed for the management of medication-induced xerostomia and how they are unlikely to be appropriate symptom management solutions.
Conclusions
Xerostomia is likely caused by multiple factors, including multiple medications. Recognizing individuals that may be suffering from this bothersome side effect and providing safe management solutions is a safe way to improve quality of life and oral health.
Key Words
Xerostomia, dry mouth, polypharmacy, elderly, drug-induced
Introduction
Documentation supports that xerostomia affects up to 46% of Americans, especially the elderly population.1 However, it is likely occurring at a much higher incidence. Medications used to treat chronic conditions that come with advanced age may need to be recognized as a potential cause. An independent risk factor for xerostomia is polypharmacy, or a patient’s use of multiple drugs (generally 5+ medications) to treat > 1 condition.2 The population of individuals aged 65 years and older have increased in the United States by one-third between 2010 and 2019.3
Untreated xerostomia may reduce a patient’s quality of life (QOL) due to symptoms such as impaired swallowing, speech, and oral hygiene. Xerostomia can lead to other complications such as dental caries, candidiasis, glossitis, impaired use of dentures, and halitosis.1 Lack of adequate xerostomia management can lead to a subsequent decline in dental and mental health.2
Studies have examined drug-induced xerostomia;3,4however, identifying management strategies that doesn’t immediately include discontinuing these medications is needed. This paper aims to compile the evidence for what commonly prescribed medications may cause xerostomia and to identify helpful management strategies that don’t necessitate automatic discontinuation of vital medications.
Medications associated with xerostomia
The Medical Expenditure Panel Survey (MEPS)5 was consulted to identify the most commonly prescribed medications in the United States. Each medication was further evaluated individually to determine incidence reporting associated with xerostomia. Table 1 is organized by clinical trial reporting of xerostomia or dry mouth. Medications listed under high incidence were medications with greater than 10% reporting of this side effect during clinical trials, medium incidence medications had reporting between 1 – 10% and low incidence had a reporting in less than 1% of the subjects in the clinical trials. When medication package inserts noted post marketing reports of xerostomia, it was classified as an ‘unknown incidence.’ It is important also to recognize that reporting of this side effect may be low because it was not a side effect specifically asked about during the trial(s). For example, some trial protocols ask patients if they are experiencing specific side effects and patients report yes or no. In this case, if xerostomia (or dry mouth) is not listed on the protocol to ask the patient, it will not be reported unless the patient specifically documents this as happening.
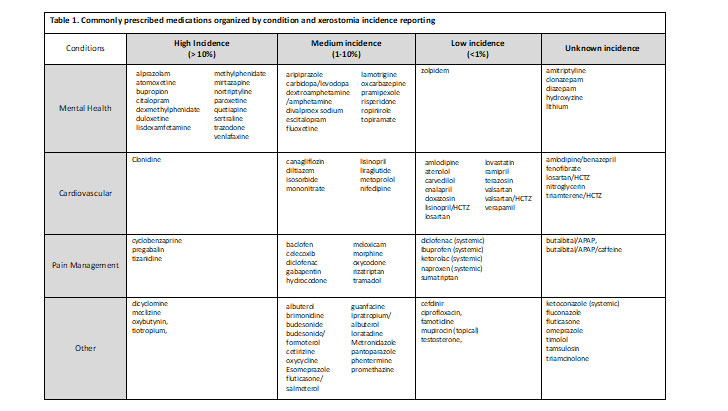
Examples of chronic conditions with medications that cause xerostomia
Mental Health
Mental health has been a growing concern for the United States population with 1 in every 5 adults diagnosed with a mental health illness.6 As of 2019, the prevalence of any mental health illness was 20.6% (51.5 million) adults aged 18 and over, while only 44.8% (23.0 million) of those diagnosed, received any form of treatment.6 More so, suicide rates have been on the rise and in 2019, suicide was the tenth leading cause of death in the U.S., claiming more than 47,500 people.7 Antidepressant medications aim to relieve the symptoms of depression (lethargy, restlessness, sleep problems, etc.) and prevent them from returning by increasing the exposure to serotonin in the brain. As provided in Table1 and Figure 1, many of these medications are chronic (used daily) and possess a medium to high incidence of causing xerostomia. While this risk may be off-putting to patients, the overall benefit of mental health medications may outweigh the risks and side effects.
Heart Disease
Claiming around 655,000 Americans each year, heart disease is the leading cause of death in the United States (and globally).8 To break that down even further, one person dies every 36 seconds due to cardiovascular disease.8 With over 126 million Americans diagnosed with cardiovascular disease (CVD), cardiovascular risk reduction strategies are imperative.9 While lifestyle modifications may lower risk of CVD, often times, medications are needed to further reduce CVD risk.8As exhibited from Table1 and Figure 1, these chronic medications may contain a medium to low incidence of xerostomia; however, the benefits of the medications far outweigh the risk.
Pain
With 20.4% of adults presenting with chronic pain in 2019, the highest impact was among adults aged 65 and over.10 Chronic pain may limit work activities, result in poor quality of life, and may even lead to poor mental health.10 While some pain medications may be temporary, there are patients that may need chronic pain medication to support activities of daily living and quality of life. As exhibited in Table 1, many pain medications pose a medium risk (1 – 10%) for xerostomia, however, pain relief is important to improve patients’ quality of life and function.
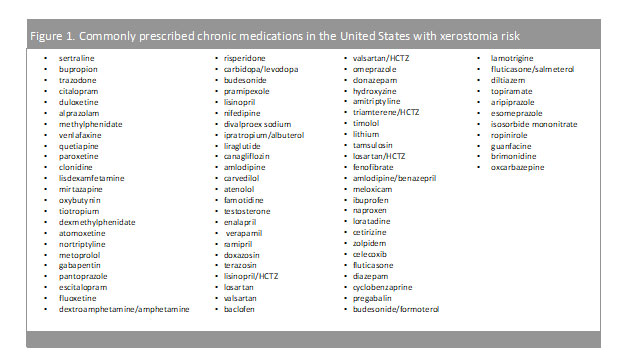
Chronic medications…stay on, or discontinue?
Patients may not associate xerostomia as the cause of certain morphologic findings they may be experiencing such as halitosis, dental caries, cracked or peeled lips, and burning mouth. Furthermore, patients may not understand why they practice good oral hygiene but still experience clinical manifestations of xerostomia. Many of the medications that may cause xerostomia are used for cardiovascular risk reduction, mental health, or pain management. Because these medications are important for reducing mortality risk and improving overall quality of life, informing a patient to stop these medications may not be ideal.
With the recognition that many medications that cause xerostomia are important for individual health and well-being, it is important to provide management solutions that do not require additional medications or discontinuing medications that are needed. Furness et al. provided a systematic review evaluating non-pharmacological methods for treating xerostomia.11 It is a commonly referenced article to highlight that non-pharmacological approaches to managing xerostomia are not effective. Of note, the non-pharmacological approaches evaluated in this excellent systematic review were acupuncture, electrostimulation and powered vs. manual toothbrushing.11 This paper is not recommending or discussing any of these approaches.
Management options for drug-induced xerostomia
Although discontinuing chronic medications that cause xerostomia is not necessarily recommended, there are preventative strategies that can be taken to reduce oral dryness. Adequate hydration and good oral hygiene will help with the symptoms of xerostomia. Regular dental visits will help with the prevention and management of dental caries, glossitis, and halitosis.1 The European League Against Rheumatism (EULAR) recommends topical therapies such as chewing gums, candies, and saliva stimulants as first line for symptoms of dry mouth, while reserving systemic treatments (pilocarpine and cevimeline) for more active/severe cases.12
Ingredients found in over-the-counter oral products that may aid with xerostomia symptoms
Cetylpyridinium Chloride (CPC)
Cetylpyridinium chloride (CPC) 0.05% is an agent found in mouthwashes that helps prevent/reduce plaque in order to prevent gingivitis or bleeding gums. Possessing anti-bacterial activity, CPC is able to bind to bacterial cells, disrupt their cell membrane integrity, eventually leading to cell death.13 CPC exhibits a well-established safety profile as well as few adverse effects. The most prominent side effect associated with CPC is teeth staining/discoloration. A study composed of 81 participants saw teeth staining in about 10.4% of patients after using CPC three times daily for 6 months.14 However, though this adverse effect was observed by the researchers, patients did not complain about this side effect in their questionnaire at the end of study.14 Given the favorable benefits of CPC and little adverse effects, a product with CPC in the ingredient list will help to prevent some complications associated with xerostomia.
Xylitol
Xylitol is a sugar alcohol found in many fruits and vegetables that can also be used as a sugar substitute. Xylitol works by disrupting mutans streptococci’s, the most common bacteria found in dental decay, energy production processes leading to cell death.15 Due to this mechanism of action, xylitol is able to increase salivary flow, decrease plaque levels, and decrease dental decay.15 However, studies have shown that about 5 to 6 grams of xylitol divided into two or three doses is needed in order to display clinical effects.16 Xylitol is in many of the OTC products and comes in different dosage forms which may make it easier to achieve this target of 5 to 6 grams per day.
Isomalt
Isomalt is a sugar substitute that is widely used in candy, gum, and oral health products. Because it does not cause the same damage to teeth as sugar, isomalt is classified as non-acidogenic as well as non-cariogenic. There has also been a study that proves that isomalt is associated with enhancing remineralization of the enamel through calcium binding.17 Isomalt also does not raise blood sugar or lipids which is beneficial for the diabetes and dyslipidemic patient populations.18
Olive oil
Xerostom® brand uses olive oil in their products due to the anti-microbial, lubrication, anti-inflammatory, and anti-caries properties.19 While the mechanism is not completely understood, olive oil seems to affect the adhesion and growth of bacteria in order to provide its anti-microbial and anti-caries activity.20 There have also been observations that show that olive oil decreases mineral loss as well as improves oral malodor.21 With these many benefits and lack of side effects, products that contain olive oil may be an attractive option for patients suffering from xerostomia.
Betaine
Betaine is a natural product found in sugar beet molasses that is commonly found in cosmetics. The main property of betaine is its ability to reduce skin-irritating effects in cosmetics and oral products (toothpastes, gels, etc.) by providing a water coating that protects cells from surfactants.22 Betaine also possess an osmo protectant effect that allows it to provide extra lubrication for patients with dry mouth.19
Sodium fluoride
Sodium fluoride is a natural element that is widely used throughout dental treatments. By acting as a catalyst for the diffusion of calcium and phosphate into the tooth, fluoride is able to make the tooth more resistant to acidity and promotes remineralization, making the teeth strong and resistant to teeth decay.23 Studies have also shown that fluoride is capable of stopping teeth decay after it has already started.24 The strong evidence provided for the anticariogenic properties of fluoride make it an efficacious option for treating the complications of xerostomia.
Alcohol/Ethanol
Mouthwashes may contain alcohol (ethanol) in their product due to the ability of alcohol to be a solvent for other ingredients, act as a preservative, and contain antiseptic properties. Compared to alcohol-free mouthwashes, studies have shown that the alcohol content improves efficacy of the product in reducing bacterial plaque and gingivitis.25
Essential oils
Essential oils (EO) have been an emerging interest in the world of dentistry as they possess antibacterial, antifungal, and antioxidant properties. EO kills bacteria by disrupting their cell walls and inhibiting enzyme activity.26 A study comparing EO-based mouthwash with chlorhexidine mouthwash, both displayed the same efficacy in terms of reductions in supragingival plaque and gingivitis.27 However, the side effect profile favored EO mouthwash due to the lack of teeth staining and calculus deposition.27
Malic acid
Although malic acid 1% has been shown to improve the dry mouth feeling and stimulate saliva production in patients with xerostomia from hypertensive medications, it is not an ideal treatment option due to adverse impact on teeth integrity with long-term use.28 Overtime this acid may lead to tooth decay, and since chronic medications are continuously taken, one can’t assume that drug-induced xerostomia would diminish with short-term malic acid therapy. Ideally management strategies would be multi-factorial and continuous.
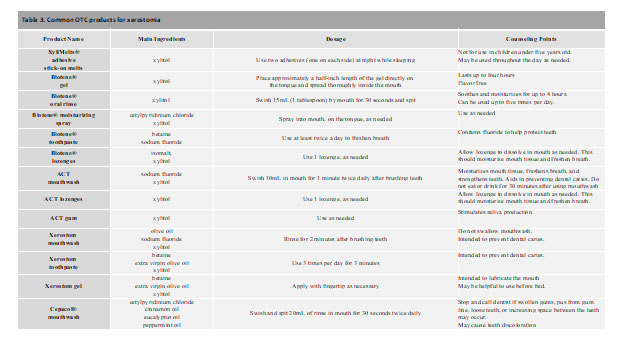
OTC product examples
Xerostom®
Xerostom® products are specifically designed to treat symptoms of dry mouth using the ingredients of olive oil, betaine, and xylitol. The product line includes toothpaste, mouth rinse, sprays and gels. Each active ingredient was included to help was a specific symptom of dry mouth: xylitol helps to prevent dental caries; olive oil is used as an oral lubricant with anti-inflammatory and anti-microbial properties; and betaine is a wetting agent that may also help with reducing skin-irritating effects that may be associated with oral products.19 When compared with patients’ various normal dry mouth routines, Xerostom® exhibited a significant increase in unstimulated whole saliva produced, improvement in mouth and tongue dryness, and a decrease in thirst.19 Patients also experienced an increase in quality-of-life issues relating to personal, physical function and pain without any reported side effects.19 Overall, Xerostom® products provide sufficient efficacy and may be appropriate for the toolbox for treating symptoms of medication-induced xerostomia.
XyliMelts®
XyliMelts® are adhesive stick-on melts that contain the active ingredient xylitol. While these adhesives can be used during both the day and while sleeping, studies have determined significant benefit in reducing morning oral discomfort, ability to swallow, and salivary production.30,31 As the main ingredient, xylitol helps with preventing dental caries and tooth decay that can be commonly associated with xerostomia. With little to no side effects, XyliMelts® are a safe and reliable option for managing symptoms of xerostomia.
Prescription medications
Pilocarpine
Pilocarpine (Salagen®) is a systemic agent FDA approved for the management of xerostomia in individuals with Sjogren’s syndrome or xerostomia due to radiation of the head and neck in the setting of cancer.33 This is a nonselective muscarinic agonist and parasympathetic medication that is dosed at 5 mg by mouth four times daily for at least 3 months.1,33 Drugs that are dosed 4 times daily are difficult to adhere to, especially if they come with a lot of side effects, as pilocarpine does. In the setting of severe Sjogren’s syndrome or when individuals are undergoing radiation to the head or neck, it may have it’s worth, but outside of these scenarios, it is not ideal. Pilocarpine works by stimulating the secretions of the exocrine glands which increases salivary flow.1,33This is needed in the conditions in which it is indicated as there is a severe deficiency of saliva production. In a study performed by Aframian et al., 45 patients were evaluated for three hours following the administration of pilocarpine. 32 Within this three-hour period, common complaints included urinary frequency, dizziness, and sweating.32 Pilocarpine should not be used in individuals with cardiovascular disease, chronic pulmonary disease (COPD), uncontrolled asthma, iritis and narrow-angle glaucoma, active gastric ulcers, and in those takingβ-adrenergic blockers (such as metoprolol and carvedilol).1,33
Cevimeline
Cevimeline (Evoxac®) is another systemic medication with FDA approval for the management of xerostomia in individuals with Sjogren’s syndrome.35 Like pilocarpine, cevimeline is a muscarinic agonist, although it is associated with fewer side effects than pilocarpine due to lack of effect on M2 receptors. The M2 receptors are mostly located in the heart, and without this site of action, cevimeline exhibits fewer cardiac side effects.34The standard dosing of cevimeline is 30mg by mouth three times daily.35Common adverse effects include dyspepsia and nausea.35 Like pilocarpine cevimeline should be avoided in individuals with COPD, uncontrolled hypertension, patients taking β-adrenergic blockers, and active gastric ulcers.1,35 Even though the side effect profile shows an advantage over pilocarpine, the precautions and risks remain.
Bethanechol
Bethanechol is an analog of acetylcholine that also possesses some muscarinic activity. Due to the resistance of destruction by cholinesterase, bethanechol results in prolonged activity. While mostly used for urinary retention, reports have demonstrated benefit of 25mg three times daily for dry mouth.36 In a study conducted by Muralidharanet.al., bethanechol was compared to pilocarpine in the treatment of xerostomia. Both drugs increased the mean salivary production, however, pilocarpine was found to be more effective of the two medications.36 The safety profiles of the two medications appeared to be similar as well as the contraindications/precautions.36 Because bethanechol is not FDA approved as a treatment for xerostomia and has exhibits less efficacy in comparion to a drug that is indicated, it is not an appropriate treatment for xerostomia.
Discussion
Recognizing that patients may be on more than one medication lending towards medication-induced xerostomia, it is appropriate to consider dry mouth management in those on many medications. Management strategies may include, (See Table 2 for product description and administration recommendations):
- Mouthwash twice daily, spray as needed throughout the day, Xylimelts® every night
- Mouthwash twice daily, followed by gel, spray as needed throughout the day, gel or Xylimelts® every night
- Mouthwash twice daily, ACT gum or lozenges throughout the day, gel or Xylimelts® every night
- Any combination of options listed above
CONCLUSION
Medication-induced xerostomia is an underrepresented condition that can decrease quality of life and health. While the number one cause of xerostomia is medication-induced, stopping medications that reduce mortality may not be a viable option. Instead, there are many OTC products that may help manage/reduce the side effects of xerostomia without adding any debilitating side effects of their own. Additionally, dentists are ideal clinicians to recognize xerostomia and evaluate if treatment measures are working sufficiently, and when management strategies may need to increase in order to prevent adverse effects associated with xerostomia.
REFERENCES
- Marcott S, Dewan K, Kwan M, Baik F, et al. Where dysphagia begins: polypharmacy and xerostomia. Fed Pract. 2020 May;37(5):234-241.
- Soto AP, Meyer SL. Oral Implications of Polypharmacy in Older Adults. Dent Clin North Am. 2021;65(2):323-343.
- Tan ECK, Lexomboon D, Sandborgh-Englund G, Haasum Y, Johnell K. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 2018;66(1):76-84.
- Shetty SR, Bhowmick S, Castelino R, Babu S. Drug induced xerostomia in elderly individuals: An institutional study. Contemp Clin Dent. 2012;3(2):173-5.
- Prescription data source: Medical Expenditure Panel Survey (MEPS) 2008-2018. Agency for Healthcare Research and Quality (AHRQ), Rockville, MD. ClinCalcDrugStats Database version 21.
- Mental Illness. National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/mental-illness. Published 2019. Accessed September 3, 2021.
- National Institute of Mental Health. https://www.nimh.nih.gov/health/statistics/suicide. Published 2019. Accessed September 3, 2021.
- Heart disease facts. Centers for Disease Control and Prevention. https://www.cdc.gov/heartdisease/facts.htm. Published September 8, 2020. Accessed September 3, 2021.
- 2021 heart disease and stroke statistics update fact sheet At-a-Glance. American Heart Association. 2021.
- Zelaya CE, Dahlhamer JM, Lucas JW, Connor, EM. Chronic pain and high-impact chronic pain among U.S. Adults, 2019. NCHS Data Brief. No. 390. 2020; Available at: https://www.cdc.gov/nchs/products/databriefs/db390.htm.
- Furness S, Bryan G, McMillan R, Worthington HV. Interventions for the management of dry mouth: non-pharmacological interventions. Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No.: CD009603.DOI: 10.1002/14651858.CD009603.pub2.
- Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al. EULAR recommendations for the management Of SJÖGREN’S syndrome with topical and systemic therapies. Annals of the Rheumatic Diseases. 2019;79(1):3-18.
- Rizwanna N. The role of cetylpyridinium chloride mouthwash in the treatment of periodontitis. International Journal of Pharmaceutical Science Invention. 2013;2(12):36-37.
- Van Leeuwen MPC, Rosema NAM, Versteeg PA, Slot DE, Van Winkelhoff AJ, Van der Weijden GA. Long-term efficacy of a 0.07% cetylpyridinium chloride mouth rinse in relation to plaque and gingivitis: a 6-month randomized, vehicle-controlled clinical trial. Int J of Dent Hygiene. 2015;13:93-103.
- Nayak PA, Nayak UA, Khandelwal V. The effect of xylitol on dental caries and oral flora. Clinical, Cosmetic and Investigational Dentistry. November 2014:89.
- Milgrom P, Ly KA, Rothen M. Xylitol and its vehicles for public health needs. Advances in Dental Research. 2009;21(1):44-47. doi:10.1177/0895937409335623
- Takatsuka T, ten cate J, Exterkate RAM. Effects of isomalt on enamel de- and remineralization, a combined in vitro pH-cycling model and in situ study. Clinical Oral Investigations. July 2008.
- Patton J, O’Brien Nabors L. Polyols: Sweet Oral Benefits. J Int Oral Health. September 2011.
- Ship JA, McCutcheon JA, Spivakovsky S, Kerr AR. Safety and effectiveness of topical dry mouth products containing olive oil, betaine, and xylitol in reducing xerostomia for polypharmacy-induced dry mouth. Journal of Oral Rehabilitation. 2007;34(10):724-732.
- Pretty IA, Gallagher MJ, Martin MV, Edgar WM, Higham SM. A study to assess the effects of a new detergent-free, olive oil formulation dentifrice in vitro and in vivo. Journal of Dentistry. 2003;31(5):327-332.
- Kensche A, Reich M, Kümmerer K, Hannig M, Hannig C. Lipids in preventive dentistry. Clinical Oral Investigations. 2012;17(3):669-685.
- Rantanen I, Tenovuo J, Pienihakkinen K, Soderling E. Effects of a betaine-containing toothpaste on subjective symptoms of dry mouth: a randomized clinical trial. The Journal of Contemporary Practice. 2003;4.
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51-59.
- Medjedovic E, Medjedovic S, Deljo D, Sukalo A. Impact of fluoride on dental health quality. Mater Sociomed. 2015;27(6):395-398.
- Werner CW, Seymour RA. Are alcohol containing mouthwashes safe? British Dental Journal. November 2009.
- Morozumi T, Kubota T, Abe D, Shimizu T, Nohno K, Yoshie H. Microbiological effect of essential oils in combination with subgingival ultrasonic instrumentation and mouth rinsing in chronic periodontitis patients. Int J Dent. 2013;2013:1-7.
- Charles CH, Mostler KM, Bartels LL, Mankodi SM. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. Journal of Clinical Periodontology. August 2004.
- Gómez-Moreno G, Guardia J, Aguilar-Salvatierra A, Cabrera-Ayala M, et al. Effectiveness of malic acid 1% in patients with xerostomia induced by antihypertensive drugs. Med Oral Patol Oral Cir Bucal. 2013 Jan 1;18(1):e49-55.
- Page RL 2nd, O’Bryant CL, Cheng D, Dow TJ, et al; American Heart Association Clinical Pharmacology and Heart Failure and Transplantation Committees of the Council on Clinical Cardiology; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016 Aug 9;134(6):e32-69.
- Burgess J. OTC Management of Dry Mouth. Clinical & Techniques. July 2016.
- vanderVen P, Burgess JA. Lubricating Adhering Discs for Relief of Oral Dryness: A literature review and report examining the use of oral adhering discs for patients experiencing xerostomia. Decisions in Dentistry. June 2020;6(6):16–18,21.
- Aframian DJ, Helcer M, Livni D, Robinson SDM, Markitziu A, Nadler C. Pilocarpine treatment in a mixed cohort of xerostomic patients. Oral diseases. 2007;13(1):88-92.
- Pilocarpine [Salagen®] package insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/020237s012lbl.pdf
- Saternos HC, Almarghalani DA, Gibson HM, et al. Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiological Genomics. 2018;50(1):1-9.
- Evoxac (cevimeline) package insert. Edison, NJ:Daiichi Sankyo Pharma Development; 2018
- Muralidharan J, Dhanraj M, Jain AR. Comparison of pilocarpine and bethanechol as effective sialogogue agents in xerostomic completely edentulous patients. Drug Inventory Today. 2018;10(6).


















