Oral drug administration through solid oral dosage forms (SODF) remains the predominant method of drug therapy in both primary and secondary healthcare settings. This is particularly pertinent in the context of an aging population, where there is compelling evidence of an increasing prevalence of clinically significant swallowing challenges, such as dysphagia.1 This trend is particularly pronounced among older individuals who are characterized by multimorbidity, frailty, and a reliance on multiple medications daily.2
Swallowing difficulties exert a detrimental influence on the administration of SODF, resulting in suboptimal adherence and inappropriate modifications such as crushing, opening, or splitting of dosage forms. Over the years, various strategies have been proposed to ameliorate the swallowing experience associated with SODF, encompassing patient administration techniques and the utilization of swallowing aids and devices.3 More recently, appropriate SODF size reductions (e.g., minitablets)4 and research on film coating materials that ease oral administration (e.g., Opadry EZ) have also been proposed. 5,6 Nonetheless, the imperative for appropriate SODF designs persists, necessitating a patient-centric drug product design foundation to establish itself as the status quo within pharmaceutical operations.7
To address it appropriately, SODF must be designed not only to promote unit dose manufacturability but also to meet the swallowability needs of the target patient population. The latter is often neglected by pharmaceutical companies during development stages, as their focus pertains to achieving suitable clinical endpoints that allow for quick market entry and return on investment.8 This holistic approach underscores the need for ongoing advancements in SODF design that optimize oral drug delivery towards patient populations with swallowing impairments. Nevertheless, with the recent launch of TaBlitz, one might be a step closer to achieving it.9
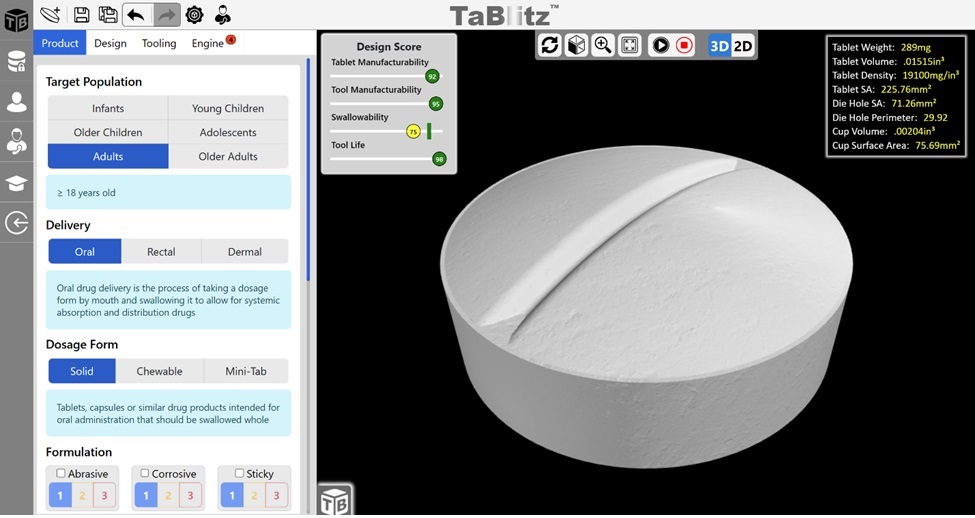
TaBlitz is an intelligent 3D design software that enables users to engineer pharmaceutical tablets in just a couple of minutes. The software focuses on streamlining standard tablet manufacturing processes by evaluating the proposed tablet design and ensuring it meets the pharmaceutical industry standards. Each design is guided by the Tableting Specification Manual (TSM) and the software’s AI design engine. The intelligence engine not only notifies users of design pitfalls that may lead to costly challenges during tablet compression (e.g., sticking, picking, capping) but also provides recommendations in situ to improve the overall tablet design (Fig.1).
Another interesting feature of TaBlitz is the “Guided Mode” function. Powered by its AI intelligence engine, the Guided Mode recommends high quality tablet designs based on minimal information given by the user. With just a few parameters input, the TaBlitz AI engine generates an array of designs that fulfill the desired tablet weight, while simultaneously ensuring compliance with industry guidelines. Each tablet design is accompanied by its design score, allowing the user to select a suitable tablet design considering their business needs. This function can also be particularly useful for early-career employees that lack the necessary experience and/or training within tablet design and manufacturing operations (Fig. 2).
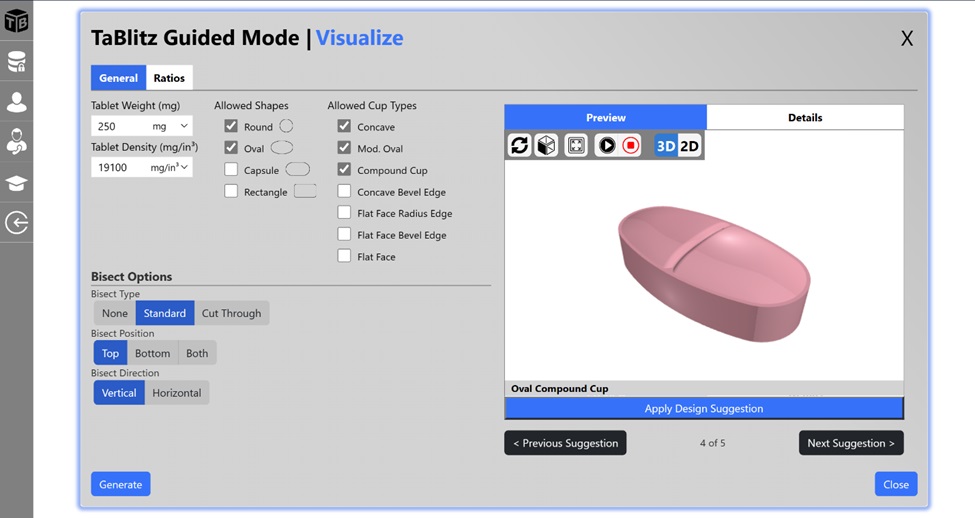
Figure 2. Overview of TaBlitz’s “Guided Mode” function (sourced from tablitz.app).
TaBlitz’s recommendations are driven by a real-time design score index comprised of 4 important metrics that help navigate the user on relevant design aspects that affect SODF manufacturing operations, tooling, and patient oral administration. The design score includes:
- Tablet manufacturability index: design score that predicts and prevents issues associated with the compression process, such as capping, sticking, picking, core erosion, etc.
- Tool manufacturability index: design score that predicts the complexity associated to the production of the tooling (e.g., punches) required to compress the engineered tablet design.
- Tool life index: design score that estimates gradual tooling wear based on the complexity of the engineered tablet design.
- Swallowability index: design score that estimates the patient’s swallowability considering the engineered tablet design.
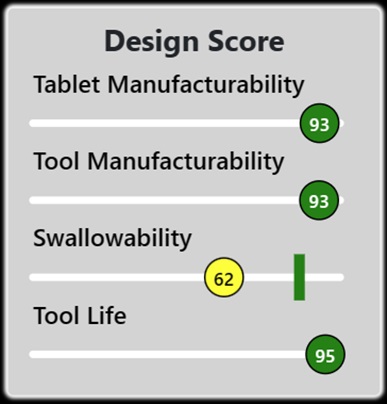
Figure 3. Overview of TaBlitz design score (sourced from tablitz.app).
A particular feature that clearly differentiates TaBlitz from any other competitors in the tablet design software space is its swallowability index. The swallowability index provides guidance to users on how their proposed tablet design will affect oral drug administration by patients at the very end of the pharmaceutical supply chain. The index score was built by applying the research findings available in the scientific literature on SODF swallowability among different patient populations. It is driven by several design factors including patient age group, SODF dimensions (e.g., size, shape, thickness), SODF weight and density, and surface coating polymer. The design of bisects is also available within the software’s tablet design toolkit, whilst the intelligence engine also suggests its inclusion in the tablet design whenever the swallowability index score is below a defined threshold. All above-described factors are embedded into the software’s tablet design interface, which altogether rewards the user with a friendly and intuitive design experience.
Compliance with oral solid dose therapies may be perceived as ordinary for some but remains a burden for many special patient populations across the globe. The underestimation of the potential impact of a tablet design on patients’ swallowability needs to be stressed during development stages. Thanks to TaBlitz’s recent launch, pharmaceutical companies can now use the software to design high quality tablets that not only allow robust manufacturing but also support patient oral administration safety and swallowability.
Adopting the TaBlitz platform in the early stages of pharmaceutical product development can help prevent costly tablet manufacturing issues and strongly supports patient centric pharmaceutical drug product design. Due to its strong capabilities, TaBlitz is set to disrupt (for the better) some aspects of the current pharmaceutical business model.
Learn how TaBlitz can address your compression challenges and streamline your tablet design experience by visiting their website at www.tablitz.us.
Nélio Drumond, PharmD, PhD
Industry Expert on Patient Centric Drug Product Design
Founder of The Pharma xCentric
https://www.pharmaxcentric.com

References
- Stegemann et al. (2012), Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int J Pharm, 430, 197-206.
- Perrie et al. (2012), The impact of ageing on the barriers to drug delivery. Control Release, 161, 389-398.
- Drumond et al. (2020), Better Medicines for Older Patients: Considerations between Patient Characteristics and Solid Oral Dosage Form Designs to Improve Swallowing Experience. Pharmaceutics, 13, 32.
- van Riet-Nales et al. (2011), The availability and age-appropriateness of medicines authorized for children in the Netherlands. British Journal of Clinical Pharmacology, 72: 465-473.
- Ershad et al. (2021), Multi-Analytical Framework to Assess the In Vitro Swallowability of Solid Oral Dosage Forms Targeting Patient Acceptability and Adherence. Pharmaceutics, 13, 411.
- Drumond et al. (2022), An evaluation of film coating materials and their predicted oro-esophageal gliding performance for solid oral dosage forms. Journal of Drug Delivery Science and Technology, 77, 103804.
- Drumond et al. (2017), Patients’ appropriateness, acceptability, usability and preferences for pharmaceutical preparations: Results from a literature review on clinical evidence. Int J Pharm, 521, 294-305.
- Steinman F. (2020), Is this the year of the patient-centric business model for healthcare? OpenText Blogs.
- Website: www.tablitz.us.



















