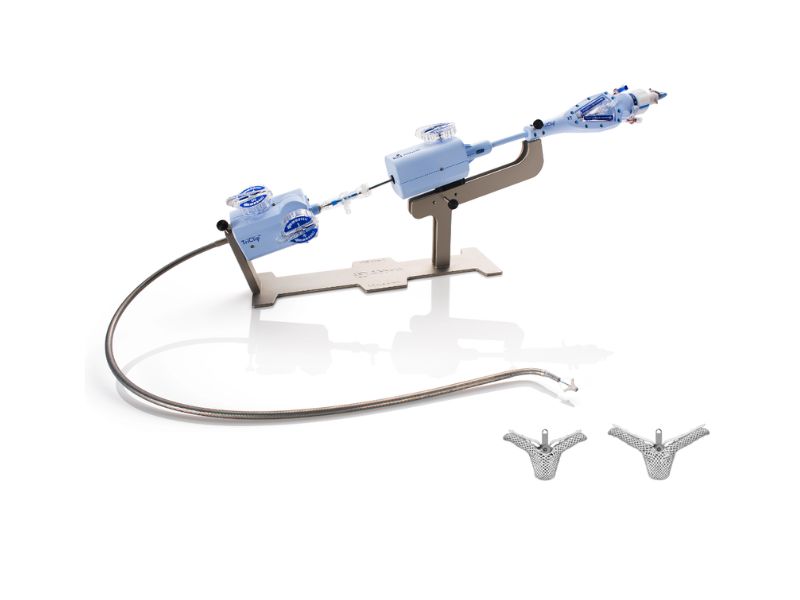
The US Food and Drug Administration has officially approved Abbott’s first-of-its-kind TriClip™ transcatheter edge-to-edge repair (TEER) system, which is designed to treat tricuspid regurgitation (TR), or a leaky tricuspid valve. To put things into context, your tricuspid valve basically bears the responsibility to control blood as it flows from the heart’s right atrium to the right ventricle. However, when this valve doesn’t close properly, it triggers a leak where the blood begins to flow backward in the heart, causing tricuspid regurgitation. As it develops, TR tends to make the heart work harder than normal, and by doing so, it causes symptoms like fatigue and shortness of breath. Furthermore, if left untreated, the condition in question can also take up the disguise of atrial fibrillation, heart failure, and ultimately, death. Now, while surgery has long been touted as an option, it isn’t an alternative for those who continue to experience symptoms despite treatment with medical therapy. Enter Abbott’s TriClip, which can improve the quality of life for patients falling under this umbrella. In practice, TriClip’s TEER technology works by clipping together a portion of the leaflets, or flaps of tissue, to repair the tricuspid valve and help blood flow in the right direction without the need for open-heart surgery. Making the option even more attractive is a fact that, on an average, people who receive TriClip only need one day in the hospital before thLifeey recover and can return home.
“The U.S. approval of TriClip is a significant advancement for people suffering from tricuspid regurgitation, a heart condition that negatively impacts their quality of life and puts them at grave risk of serious health issues,” said Paul Sorajja, director of the Center for Valve and Structural Heart Disease for the Minneapolis Heart Institute at Abbott Northwestern Hospital, and lead investigator of the trial validating the TriClip.
Talk about the trial, Abbott’s latest brainchild got the regulatory nod only after it presented its findings from TRILUMINATE Pivotal trial, the world’s first randomized, controlled clinical study to evaluate the safety and effectiveness of the TriClip system. This evaluation was conducted in comparison to medical therapy among people with severe TR who are at intermediate or greater risk for open-heart surgery. Going by the published results, nearly 90% of patients who received the TriClip system experienced a marked improvement in their TR grade, reducing from severe or higher to moderate, or even less, in 30 days. These patients also sustained the stated reduction for one whole year. Furthermore, the trial revealed a highly favorable safety profile, given almost 98% of patients emerged as free of major adverse events through 30 days, while simultaneously experiencing a significant improvement in quality of life.
Another detail worth a mention here is rooted in the brand-new TriClip’s underlying clip-based technology which is similar to Abbott’s leading MitraClip™ device. The MitraClip device, on its part, has already treated more than 200,000 people with leaky mitral valves (mitral regurgitation). It was, though, specifically designed to treat the tricuspid valve’s complex anatomy.
“This approval helps address a treatment gap for people with tricuspid regurgitation who previously had few options to treat a disease that adversely impacted their daily lives and could lead to other deadly conditions,” said Sandra Lesenfants, senior vice president of Abbott’s structural heart business. “With the addition of TriClip to our broad structural heart therapy offerings in the U.S., we are continuing to bring meaningful, life-enhancing benefits to patients with cardiovascular conditions.”